46 Propagation of the Signal
Learning Objectives
By the end of this section, you will be able to do the following:
- Explain how the binding of a ligand initiates signal transduction throughout a cell
- Recognize the role of phosphorylation in the transmission of intracellular signals
- Evaluate the role of second messengers in signal transmission
Once a ligand binds to a receptor, the signal is transmitted through the membrane and into the cytoplasm. Continuation of a signal in this manner is called signal transduction. Signal transduction only occurs with cell-surface receptors, which cannot interact with most components of the cell such as DNA. Only internal receptors are able to interact directly with DNA in the nucleus to initiate protein synthesis.
When a ligand binds to its receptor, conformational changes occur that affect the receptor’s intracellular domain. Conformational changes of the extracellular domain upon ligand binding can propagate through the membrane region of the receptor and lead to activation of the intracellular domain or its associated proteins. In some cases, binding of the ligand causes dimerization of the receptor, which means that two receptors bind to each other to form a stable complex called a dimer. A dimer is a chemical compound formed when two molecules (often identical) join together. The binding of the receptors in this manner enables their intracellular domains to come into close contact and activate each other.
Binding Initiates a Signaling Pathway
After the ligand binds to the cell-surface receptor, the activation of the receptor’s intracellular components sets off a chain of events that is called a signaling pathway, sometimes called a signaling cascade. In a signaling pathway, second messengers–enzymes–and activated proteins interact with specific proteins, which are in turn activated in a chain reaction that eventually leads to a change in the cell’s environment ((Figure)), such as an increase in metabolism or specific gene expression. The events in the cascade occur in a series, much like a current flows in a river. Interactions that occur before a certain point are defined as upstream events, and events after that point are called downstream events.
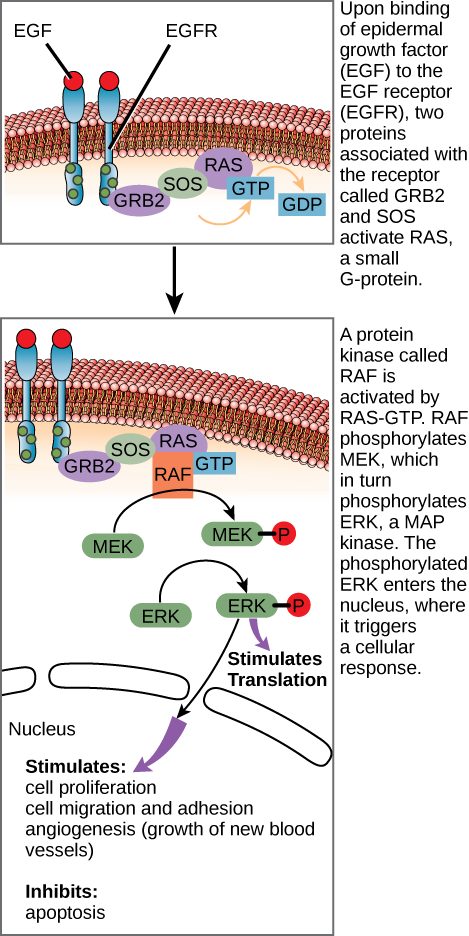
In certain cancers, the GTPase activity of the RAS G-protein is inhibited. This means that the RAS protein can no longer hydrolyze GTP into GDP. What effect would this have on downstream cellular events?
You can see that signaling pathways can get very complicated very quickly because most cellular proteins can affect different downstream events, depending on the conditions within the cell. A single pathway can branch off toward different endpoints based on the interplay between two or more signaling pathways, and the same ligands are often used to initiate different signals in different cell types. This variation in response is due to differences in protein expression in different cell types. Another complicating element is signal integration of the pathways, in which signals from two or more different cell-surface receptors merge to activate the same response in the cell. This process can ensure that multiple external requirements are met before a cell commits to a specific response.
The effects of extracellular signals can also be amplified by enzymatic cascades. At the initiation of the signal, a single ligand binds to a single receptor. However, activation of a receptor-linked enzyme can activate many copies of a component of the signaling cascade, which amplifies the signal.
Observe an animation of cell signaling at this site.
Methods of Intracellular Signaling
The induction of a signaling pathway depends on the modification of a cellular component by an enzyme. There are numerous enzymatic modifications that can occur, and they are recognized in turn by the next component downstream. The following are some of the more common events in intracellular signaling.
Phosphorylation
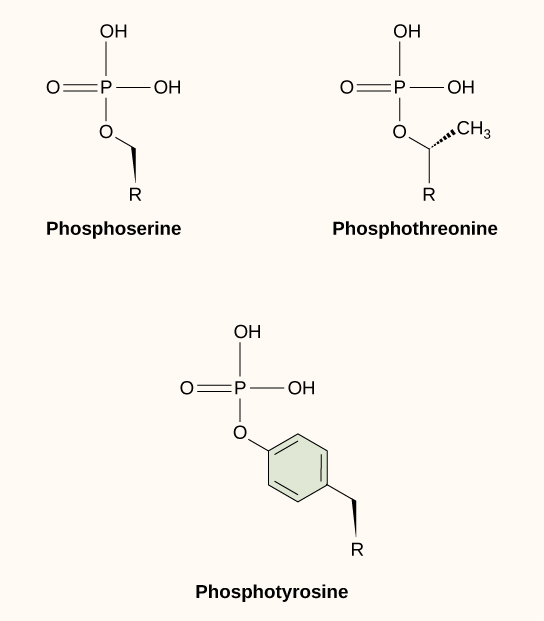
One of the most common chemical modifications that occurs in signaling pathways is the addition of a phosphate group (PO4–3) to a molecule such as a protein in a process called phosphorylation. The phosphate can be added to a nucleotide such as GMP to form GDP or GTP. Phosphates are also often added to serine, threonine, and tyrosine residues of proteins, where they replace the hydroxyl group of the amino acid ((Figure)). The transfer of the phosphate is catalyzed by an enzyme called a kinase. Various kinases are named for the substrate they phosphorylate. Phosphorylation of serine and threonine residues often activates enzymes. Phosphorylation of tyrosine residues can either affect the activity of an enzyme or create a binding site that interacts with downstream components in the signaling cascade. Phosphorylation may activate or inactivate enzymes, and the reversal of phosphorylation, dephosphorylation by a phosphatase, will reverse the effect.
Second Messengers
Second messengers are small molecules that propagate a signal after it has been initiated by the binding of the signaling molecule to the receptor. These molecules help to spread a signal through the cytoplasm by altering the behavior of certain cellular proteins.
Calcium ion is a widely used second messenger. The free concentration of calcium ions (Ca2+) within a cell is very low because ion pumps in the plasma membrane continuously remove it by using adenosine-5′-triphosphate (ATP). For signaling purposes, Ca2+ is stored in cytoplasmic vesicles, such as the endoplasmic reticulum, or accessed from outside the cell. When signaling occurs, ligand-gated calcium ion channels allow the higher levels of Ca2+ that are present outside the cell (or in intracellular storage compartments) to flow into the cytoplasm, which raises the concentration of cytoplasmic Ca2+. The response to the increase in Ca2+ varies and depends on the cell type involved. For example, in the β-cells of the pancreas, Ca2+ signaling leads to the release of insulin, and in muscle cells, an increase in Ca2+ leads to muscle contractions.
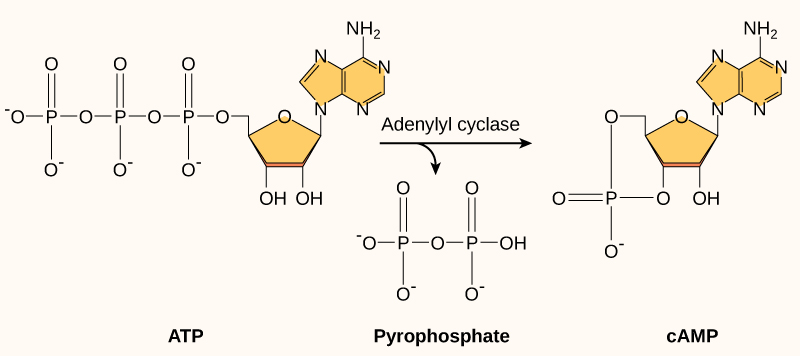
Another second messenger utilized in many different cell types is cyclic AMP (cAMP). Cyclic AMP is synthesized by the enzyme adenylyl cyclase from ATP ((Figure)). The main role of cAMP in cells is to bind to and activate an enzyme called cAMP-dependent kinase (A-kinase). A-kinase regulates many vital metabolic pathways: It phosphorylates serine and threonine residues of its target proteins, activating them in the process. A-kinase is found in many different types of cells, and the target proteins in each kind of cell are different. Differences give rise to the variation of the responses to cAMP in different cells.
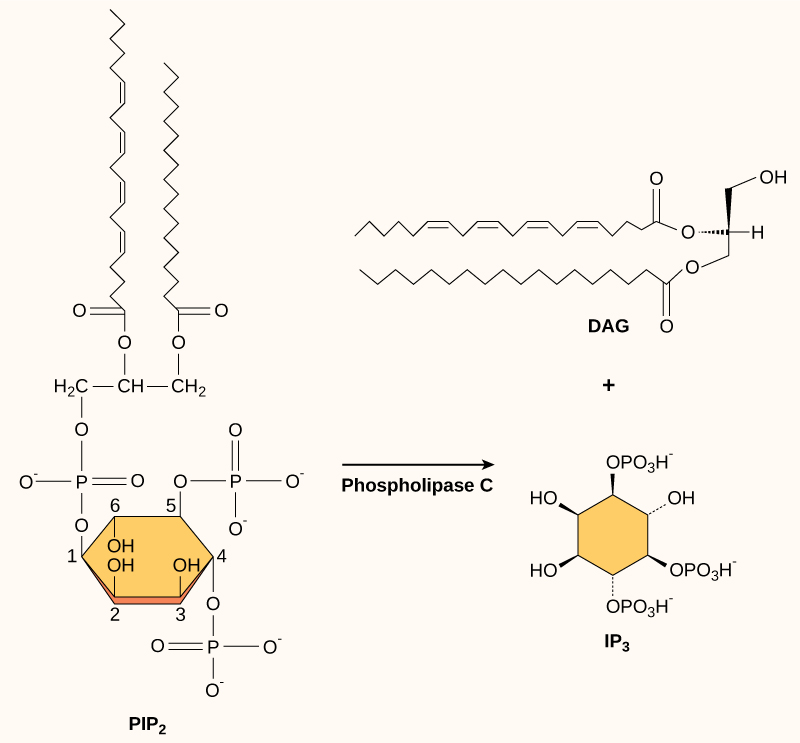
Present in small concentrations in the plasma membrane, inositol phospholipids are lipids that can also be converted into second messengers. Because these molecules are membrane components, they are located near membrane-bound receptors and can easily interact with them. Phosphatidylinositol (PI) is the main phospholipid that plays a role in cellular signaling. Enzymes known as kinases phosphorylate PI to form PI-phosphate (PIP) and PI-bisphosphate (PIP2).
The enzyme phospholipase C cleaves PIP2 to form diacylglycerol (DAG) and inositol triphosphate (IP3) ((Figure)). These products of the cleavage of PIP2 serve as second messengers. Diacylglycerol (DAG) remains in the plasma membrane and activates protein kinase C (PKC), which then phosphorylates serine and threonine residues in its target proteins. IP3 diffuses into the cytoplasm and binds to ligand-gated calcium channels in the endoplasmic reticulum to release Ca2+ that continues the signal cascade.
Section Summary
Ligand binding to the receptor allows for signal transduction through the cell. The chain of events that conveys the signal through the cell is called a signaling pathway or cascade. Signaling pathways are often very complex because of the interplay between different proteins. A major component of cell signaling cascades is the phosphorylation of molecules by enzymes known as kinases. Phosphorylation adds a phosphate group to serine, threonine, and tyrosine residues in a protein, changing their shapes, and activating or inactivating the protein. Small molecules like nucleotides can also be phosphorylated. Second messengers are small, non-protein molecules that are used to transmit a signal within a cell. Some examples of second messengers are calcium ions (Ca2+), cyclic AMP (cAMP), diacylglycerol (DAG), and inositol triphosphate (IP3).
Visual Connection Questions
(Figure) In certain cancers, the GTPase activity of the RAS G-protein is inhibited. This means that the RAS protein can no longer hydrolyze GTP into GDP. What effect would this have on downstream cellular events?
(Figure) ERK would become permanently activated, resulting in cell proliferation, migration, adhesion, and the growth of new blood vessels. Apoptosis would be inhibited.
Review Questions
Where do DAG and IP3 originate?
- They are formed by phosphorylation of cAMP.
- They are ligands expressed by signaling cells.
- They are hormones that diffuse through the plasma membrane to stimulate protein production.
- They are the cleavage products of the inositol phospholipid, PIP2.
D
What property enables the residues of the amino acids serine, threonine, and tyrosine to be phosphorylated?
- They are polar.
- They are non-polar.
- They contain a hydroxyl group.
- They occur more frequently in the amino acid sequence of signaling proteins.
C
Histamine binds to the H1 G-protein-linked receptor to initiate the itchiness and airway constriction associated with an allergic response. If a mutation in the associated G-protein’s alpha subunit prevented the hydrolysis of GTP how would the allergic response change?
- More severe allergic response compared to normal G-protein signaling.
- Less severe allergic response compared to normal G-protein signaling.
- No allergic response.
- No change compared to normal G-protein signaling.
A
A scientist observes a mutation in the transmembrane region of EGFR that eliminates its ability to be stabilized by binding interactions during dimerization after ligand binding. Which hypothesis regarding the effect of this mutation on EGF signaling is most likely to be correct?
- EGF signaling cascades would be active for longer in the cell.
- EGF signaling cascades would be active for a shorter period of time in the cell.
- EGF signaling cascades would not occur.
- EGF signaling would be unaffected.
B
Critical Thinking Questions
The same second messengers are used in many different cells, but the response to second messengers is different in each cell. How is this possible?
Different cells produce different proteins, including cell-surface receptors and signaling pathway components. Therefore, they respond to different ligands, and the second messengers activate different pathways. Signal integration can also change the end result of signaling.
What would happen if the intracellular domain of a cell-surface receptor was switched with the domain from another receptor?
The binding of the ligand to the extracellular domain would activate the pathway normally activated by the receptor donating the intracellular domain.
If a cell developed a mutation in its MAP2K1 gene (encodes the MEK protein) that prevented MEK from being recognized by phosphatases, how would the EGFR signaling cascade and the cell’s behavior change?
EGF binding to EGFR initiates a signaling cascade that activates protein kinases through phosphorylation. Active Raf phosphorylates MEK, activating MEK’s kinase activity. If MEK cannot be dephosphorylated, the signaling cascade downstream of MEK will continue to be active after the EGF signal is gone. Therefore, the cell will continue to proliferate and be resistant to cell death (apoptosis).
Glossary
- cyclic AMP (cAMP)
- second messenger that is derived from ATP
- cyclic AMP-dependent kinase
- (also, protein kinase A, or PKA) kinase that is activated by binding to cAMP
- diacylglycerol (DAG)
- cleavage product of PIP2 that is used for signaling within the plasma membrane
- dimer
- chemical compound formed when two molecules join together
- dimerization
- (of receptor proteins) interaction of two receptor proteins to form a functional complex called a dimer
- inositol phospholipid
- lipid present at small concentrations in the plasma membrane that is converted into a second messenger; it has inositol (a carbohydrate) as its hydrophilic head group
- inositol triphosphate (IP3)
- cleavage product of PIP2 that is used for signaling within the cell
- kinase
- enzyme that catalyzes the transfer of a phosphate group from ATP to another molecule
- second messenger
- small, non-protein molecule that propagates a signal within the cell after activation of a receptor causes its release
- signal integration
- interaction of signals from two or more different cell-surface receptors that merge to activate the same response in the cell
- signal transduction
- propagation of the signal through the cytoplasm (and sometimes also the nucleus) of the cell
- signaling pathway
- (also signaling cascade) chain of events that occurs in the cytoplasm of the cell to propagate the signal from the plasma membrane to produce a response

